If you are diagnosed with ovarian cancer this is for you.
A new cancer vaccine, TPIV 200, is a multi-epitope peptide cancer vaccine that targets Folate Receptor Alpha. Memorial Sloan Kettering Cancer Center in New York City and Mayo Clinic are the lead cancer centers on this new treatment, and Hawai’i patients can get TPIV-200 treatment. Call my office if you need help.
Ninety percent (90%) of ovarian cancer cells overexpress Folate Receptor Alpha, which is also overexpressed in multiple cancers.
We are delighted to bring a leading T-cell vaccine platform into this combination study and to work with AstraZeneca/Medimmune and Sloan Kettering in a patient population that is in dire need of an effective treatment. This study is part of a larger Phase II strategy for TPIV 200 that is designed to greatly increase our understanding of the vaccine while providing clinical evidence of efficacy.
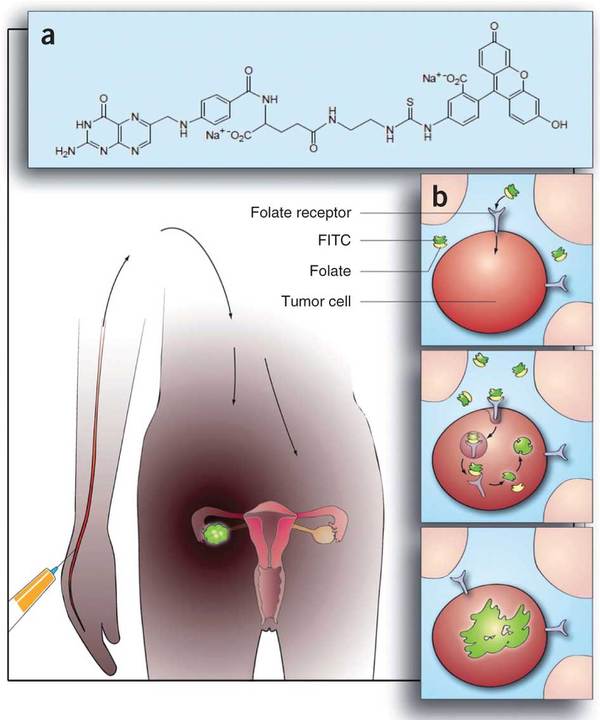
In Phase I clinical studies conducted at the Mayo Clinic in patients with breast and ovarian cancer, this vaccine was shown to be safe and well tolerated and was able to give cellular immune responses in 20 out of 21 evaluable patients.
On April 21, 2016 Memorial Sloane Kettering Cancer Center in New York City announced a new clinical trial of TPIV-200 for ovarian cancer.
Cancer vaccine and checkpoint inhibitor combination to be evaluated in platinum-resistant ovarian cancer patients
TapImmune,Inc. (TPIV), a clinical-stage immunology-oncology company specializing in the development of innovative peptide and gene-based immuno-therapeutics and vaccines for the treatment of cancer and metastatic disease, announced today plans to initiate a Phase 2 trial of its cancer vaccine, TPIV 200, a multi-epitope anti-folate receptor vaccine (FR), in combination with Astra Zeneca (NYSE: AZN) durvalumab (MEDI4736), an anti-PD-L1 antibody, in patients with platinum-resistant ovarian cancer. The study will commence in the second quarter of 2016 at Memorial Sloan Kettering Cancer Center in New York and will be led by Jason Konner, M.D. as Principal Investigator.
The two Companies will share clinical costs, while TapImmune will supply TPIV 200 and MedImmune will supply Durvalumab (MED14736) for the trials. TapImmune recently obtained Orphan Drug Designation for TPIV 200 in ovarian cancer from the U.S. Food and Drug Administration.
This single arm Phase 2 trial will include 40 women with high-grade ovarian, tubal, or primary peritoneal carcinomas, who have progressed within 6 months of their most recent platinum chemotherapy. The primary objective of the study is to determine the effectiveness of the combination by measuring Overall Response Rate [ORR = Complete Response (CR) + Partial Response (PR)] by RECIST and Progression Free Survival (PFS) rate at 6 months. Secondary endpoints will be safety and immune and correlation of FR-specific immune responses with clinical efficacy.
“This collaboration is a significant event for TapImmune,” stated Dr. Glynn Wilson, Chairman and CEO of TapImmune. “We are delighted to bring a leading T-cell vaccine platform into this combination study and to work with AstraZeneca/Medimmune and Sloan Kettering in a patient population that is in dire need of an effective treatment.”
“This study is part of a larger Phase 2 strategy for TPIV 200 that is designed to greatly increase our understanding of the vaccine while providing clinical evidence of efficacy,” Dr. Wilson added.
A woman diagnosed with ovarian cancer can get the best standard cancer treatments available for ovarian cancer in Honolulu, at the Queen’s Medical Center Cancer Center which is associated with the world famous M. D. Anderson Cancer Center in Houston, Texas. The M.D. Anderson research into immunotherapy for cancer _ cancer vaccines _ is the best in the world.
But for TPIV-200 treatment of ovarian cancer I recommend going to MSKCC in New York City and being worked up by their research team on ovarian cancer. Usually they will work with Honolulu oncologists so the woman can get her TPIV-200 in Honolulu. By getting a work-up at MSKCC the patient will have over-sight from the best doctors in the world. Mayo Clinic is also a great choice.
Hawai‘i women diagnosed with ovarian cancer should look into these new and better treatment options. The statistics at that most ovarian cancer patients survive for 5 years. I believe that you can do better. But you must take your care into your own hands and get ready for these new treatments if your initial treatment doesn’t work.Call me (808-753-0290) if you want help in finding your way through the treatment maze.
– Wayne






